It is well known that men and women have markedly different physiology. For instance, women have a greater percentage of slow-twitch muscle fibers when compared to men. Alternatively, men have considerably more fast-twitch muscle fibers than women. Additionally, there are significant sex differences in the endocrine, cardiovascular and other systems. Therefore, it is somewhat paradoxical that most exercise guidelines for men and women are basically the same. This article highlights key physiological differences between women and men, and provides health and fitness professionals with important training recommendations that account for these sex differences and help to optimize training effectiveness for both male and female clients.
Sex Differences in Muscle Fiber Types
Skeletal muscle is divided into two types of fibers: type I (slow-twitch) and type II (fast-twitch). Type I fibers are more efficient than type II fibers at using oxygen to create adenosine triphosphate (ATP), the body’s source of energy. This is ideal in endurance exercise. By contrast, type II fibers have 10 to 20 percent greater contractile force than type I fibers (Bottinelli, 1994). Type II fibers are ideal for activities requiring speed, such as sprinting, or high-force movements, such as weight lifting. The energy systems that correspond with each muscle fiber type also differ; while type I fibers predominantly use mitochondrial respiration, type II fibers predominantly use glycolysis. When studying the rate of fatigue of each muscle fiber, type I fibers are more resistant to fatigue than type II fibers.
Research has shown that women have a higher proportion of type I skeletal muscle fibers (Hicks, Kent-Braun and Ditor, 2001; Simoneau and Bouchard, 1989; Brooks and Engel, 1969). Conversely, research shows that type II muscle fibers are more prevalent than type I in men (Komi and Karlsson, 1978). The differences found in muscle-fiber proportion between men and women most certainly contribute to varying performance capabilities. For example, studies show that women have a greater resistance to skeletal muscle fatigue when compared to men (Salvador et al., 2009; Clark et al., 2005; Hunter et al., 2004). It has been suggested that the primary mechanism underpinning these fatigue-resistance differences is the sex-specific differences in type I fibers. By contrast, research also shows that men have a greater overall power output than women (Thomas et al., 2007). The assumed mechanism behind this is that men have a higher proportion of type II muscle fibers relative to women.
Sex Differences in Substrate Utilization
For a human body to perform any type of exercise, energy—in the form of ATP—is needed, and is manufactured by three systems: the phosphagen system, glycolysis and mitochondrial respiration. These three energy systems utilize different forms of fuel, also known as substrates, and produce ATP through different chemical processes. The common substrates are phosphocreatine, carbohydrates (CHO) in the form of glucose or glycogen, and lipids in the form of free fatty acids. The chemical processes for each of the energy systems vary in complexity.
The phosphagen system is the simplest chemical reaction and the quickest source of ATP. Glycolysis requires the use of one or two molecules of ATP (depending if the substrate is glucose or glycogen) and can yield two or three molecules of ATP. Although the chemical reaction of glycolysis is not as quick as the phosphagen system and has an ATP cost, it is still a rapid source of energy. Mitochondrial respiration yields the greatest number of ATP molecules, but the rate of production is considerably slower than the phosphagen system or glycolysis (Porcari, Bryant and Comana, 2015). Mitochondrial respiration can utilize both lipids and CHO as substrates (i.e., fuel sources). Mitochondrial respiration, when using CHO as a fuel source, has a faster ATP production rate, relative to when lipids are used as the fuel source.
There are a number of key differences between men and women with respect to substrate utilization. For example, one study reported that men had a 15 to 32 percent higher glycolytic enzymatic capacity when compared to women. The same study also found that women had a 15 percent higher capacity for ß-oxidation (i.e., burning fat) relative to men (Green, Fraser and Ranney, 1984). Moreover, research has repeatedly shown that women demonstrate a 4 to 5 percent lower respiratory exchange ratio (RER) than men during submaximal endurance exercise (Tarnopolsky, 2000, Tarnopolsky et al., 1997, Tarnopolsky et al., 1995). Collectively, these findings demonstrate that women are generally more reliant on lipids as a fuel source, whereas men tend to be more dependent on CHO as a substrate for metabolism. These sex differences in substrate selection can in part be explained by the dissimilarities in the distribution of muscle fibers mentioned in the previous section.
Sex Differences in Hormone Concentrations
Two principal hormones upregulated in the body during prolonged exercise are the catecholamines: epinephrine and norepinephrine. Produced in the sympathetic nerve fibers and the adrenal medulla, these two hormones play a major role in oxygen and energetic substrate transportation to active skeletal muscles. Catecholamines have been shown to stimulate respiratory, cardiac, metabolic and thermoregulatory functions (Zouhal et al., 2008).
Sex is a key factor influencing epinephrine and norepinephrine concentrations at rest and during exercise. In a study observing plasma epinephrine concentrations in response to a set of 10 x 6-second sprints on a non-motorized treadmill, it was found that there was a nearly threefold greater concentration in men compared to women (Brooks et al., 1990). Likewise, 25 to 50 percent higher epinephrine and norepinephrine concentrations were found in training-matched men when compared to women during repeated 6-second supramaximal running bouts (Gratas-Delamarche et al., 1994).
It is clear that a high capacity to secrete these hormones represent an advantage in competitive sports due to their role in regulating skeletal muscle glycogenolysis (Kjaer, 1998). In fact, a greater catecholamine concentration might be one possible explanation for the higher glycolytic capacity found in men relative to women. Another key hormone that differs greatly in men and women is the estrogen sex hormone (17-ß-estradiol). Indeed, women have significantly higher concentrations of 17-ß-estradiol when compared to their men counterparts. During a women’s menstrual cycle, 17-ß-estradiol concentrations will also fluctuate, with lower levels occurring during the follicular phase and higher levels occurring during the luteal phase. Higher concentrations of 17-ß-estradiol are positively correlated with increased lipid oxidation and a concomitant sparing of muscle glycogen (Rooney et al., 1993; Kendrick and Ellis, 1991). As stated above, lipid utilization regenerates ATP at a slower rate relative to CHO oxidation. In summary, men have higher catecholamine concentrations and lower levels of 17-ß-estradiol, whereas women have lower catecholamine concentrations and higher levels of 17-ß-estradiol.
Sex Differences in Body Composition and Thermoregulation?
Why are men and women typically shaped differently? This can be attributed, in part, to the fact that men and women differ substantially in the concentrations of the sex hormones 17-ß-estradiol and testosterone. For example, women have a fivefold greater plasma concentration of 17-ß-estradiol, while men have a nearly 15-fold higher plasma concentration of testosterone (Garaulet et al., 2000). In turn, it has been demonstrated that testosterone levels are significantly correlated with body composition values in men; in a similar manner, 17-ß-estradiol levels are significantly related to abdominal obesity in women. Additionally, it is well known that (on average) men have lower body-fat percentages when compared to women, and that women typically store body fat peripherally and subcutaneously rather than around the abdominal region like men.
The differences in body composition and adipose tissue distribution underpin important sex-specific differences in thermoregulatory capacity. Interestingly, research has demonstrated that in a hot environment, men begin to sweat at a lower core temperature, more quickly and at a greater magnitude relative to women. One study had men and women pedal on a cycle ergometer that was immersed in 82.4°F (28°C) water for 100 minutes at an intensity of 60 percent maximal oxygen uptake. Researchers found that men began to sweat at a core temperature of 98.4°F (36.9°C), while women did not begin to sweat until a significantly higher core temperature of 99°F (37.2°C) (Anderson, Ward and Mekjavic, 1995).
Additionally, researchers have observed men, post-ovulation women and pre-ovulation women in a temperature-controlled chamber for 90 minutes and measured the onset of sweating. It was reported that men began to sweat at 14 minutes, post-ovulation women began to sweat at 17 minutes and pre-ovulation women began to sweat at 24 minutes (Bittel and Henane, 1975). In another study, subjects performed a 90-minute indoor cycling bout, drank water ad libitum and were measured for rate and amount of sweat loss. It was found that men had a twofold higher rate of sweat loss and twofold greater overall magnitude of sweat loss when compared to women (Hazelhurst and Claassen, 2006). In conclusion, men and women differ in body composition, distribution of adipose tissue and thermoregulatory capacity.
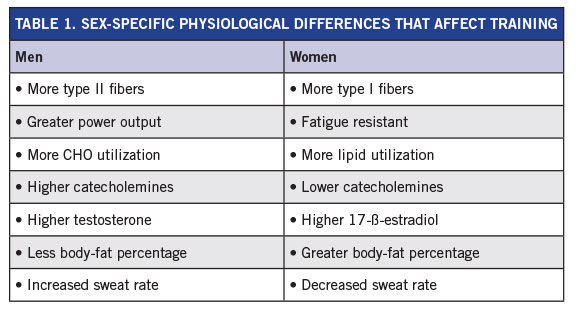
Table 1 summarizes the major physiological differences between women and men that can affect training recommendations.
Next, let’s look at several practices aimed at enhancing training effectiveness in male and female clients, given their different physiological profiles.
1. Establish Realistic Goals for Both Male and Female Clients
Substantial public health efforts have recently been aimed at promoting and increasing levels of physical activity. Regrettably, the prevalence of physical inactivity remains too high. One major area of concern is that long-term maintenance of regular exercise remains poor, with attrition rates for structured exercise programs ranging from 9 to 87 percent. Simply put, many clients are able to successfully initiate an exercise program, but fail to adhere to regular exercise throughout the lifespan.
There are numerous strategies you may elect to employ with your clients to enhance exercise adherence, including identifying individualized and achievable exercise-program goals. However, implementation of this practical recommendation requires that you have an appreciation of sex-specific differences in maximal cardiorespiratory and muscular fitness capacity and other key cardiometabolic risk factors dissimilarities. Without this knowledge, you run the risk of establishing unrealistic goals for both your male and female clients.
Primary Differences to Account for When Making Practical Recommendations:
- Compared to men, women have higher high-density lipoprotein (HDL) cholesterol levels (on average 10 points).
- Compared to men, women have lower blood-pressure levels (on average 10 mmHg).
- Relative to men, women have higher body-fat percentage levels (on average 5 to 8 percent).
- Relative to women, men have 10 to 20 percent higher cardiorespiratory levels.
- Relative to women, men have 30 to 50 percent greater upper- and lower-body strength measures.
2. Men Need More Recovery Time Than Women Do
Recovery from exercise training is a vital component of the overall exercise program, and paramount for performance and continued improvement. If the rate of recovery is suitable, higher training volumes and intensities are possible without the detrimental effects of overtraining. Understanding the physiological concept of recovery is essential for designing effective training programs.
Nevertheless, considerable individual variability exists within the recovery process due to training status (trained vs. untrained), factors of fatigue and sex differences. As previously discusses, there are notable physiological differences between men and women with respect to fatigue. Indeed, differences in skeletal muscle fiber type, substrate utilization and various hormone concentrations are collectively responsible for women being more resistant to fatigue when compared to men. This physiological reality should be considered when making recovery recommendations.
Primary Differences to Account for When Making Practical Recommendations:
- Men will likely require more rest between interval bouts when compared to women. For instance, if male and female clients are each performing 8 x 60-second intervals bouts, the female client may be fully recovered between bouts after two minutes of rest, while the male client might require an additional 30 seconds of rest.
- Men will also likely require more recovery time between sets of resistance training when compared to their female counterparts. A similar sex-specific discrepancy for rest periods as just described between interval bouts should also exist between resistance-training sets. Although there is no specific recommendation, evidence suggests that men may require anywhere between 25 and 50 percent additional recovery time.
- Men may require an additional day of recovery following higher-intensity training sessions. For instance, a male client may need three days to recover following an interval session, whereas a female might be fully recovered after only two days of moderate-intensity training.
- As noted earlier, relative to men, women typically utilize more lipid and less muscle glycogen during submaximal exercise. Accordingly, during the postexercise timeframe, it is more likely that men will have a greater requirement for CHO consumption to replenish depleted muscle glycogen stores. More information on specific strategies for postexercise CHO consumption can be found here.
3. I.T.T. Recommendations Should Be Different for Women and Men
Exercise training can be optimized by correctly managing the various components surrounding the exercise program (American College of Sports Medicine, 2014). To help ensure both your male and female clients achieve their health- and fitness-related goals, plan an appropriate F.I.T.T. (frequency, intensity, time, type) that is sex-specific.
Primary Differences to Account for When Making Practical Recommendations:
-
Frequency: Men may require fewer high-intensity sessions each week relative to women due to fatigability differences.
- Intensity: Although relative training intensity guidelines [e.g., percent heart-rate reserve or percent one-repetition maximum (1-RM)] will be similar, it is critical to understand that due to differences in maximal cardiorespiratory and muscular fitness levels, the absolute workloads performed will differ accordingly between men and women.
- Time: Women may have more potential to perform daily training that is longer in duration due to a greater reliance on lipid relative to CHO (i.e., muscle glycogen). Additionally, because women recover faster, as noted earlier, they may find it easier to perform two training sessions per day.
- Type: Training modalities are not likely to differ considerably between men and women. However, because women are less heat tolerant when compared to men due to lower sweat rates and higher percent body fat, the environmental conditions of the exercise setting will be important for safety reasons. For example, if exercise is to be performed outdoors in hot and/or humid conditions, proper attire and hydration will be particularly critical for women.
References
American College of Sports Medicine (2014). ACSM’s Guidelines for Exercise Testing and Prescription (9th ed.). Baltimore: Wolters Kluwer/Lippincott Williams & Wilkins.
Anderson, G.S., Ward, R. and Mekjavic, I.B. (1995). Gender differences in physiological reactions to thermal stress. European Journal of Applied Physiology and Occupational Physiology, 71, 95-101.
Bittel, J. and Henane, R. (1975). Comparison of thermal exchanges in men and women under neutral and hot conditions. Journal of Physiology, 250, 475-489.
Bottinelli, R. et al. (1994). Myofibrillar ATPase activity during isometric contraction and isomyosin composition in rat single skinned muscle fibres. Journal of Physiology, 481, 663-675.
Brooks, M.H. and Engel, W.K. (1969). The histographic analysis of human muscle biopsies with regard to fiber types: I. Adult male and female. Neurology, 19, 221-233.
Brooks, S. et al. (1990). The hormonal responses to repetitive brief maximal exercise in humans. European Journal of Applied Physiology and Occupational Physiology, 60, 144-148.
Clark, B.C. et al. (2005). Sex differences in muscle fatigability and activation patterns of the human quadriceps femoris. European Journal of Applied Physiology, 94, 196-206.
Garaulet, M. et al. (2000). Anthropometric, computed tomography and fat cell data in an obese population: Relationship with insulin, leptin, tumor necrosis factor-alpha, sex hormone-binding globulin and sex hormones. European Journal of Endocrinology, 143, 657-666.
Gratas-Delamarche, A. et al. (1994). Lactate and catecholamine responses in male and female sprinters during a Wingate test. European Journal of Applied Physiology, 68, 362-366.
Green, H.J., Fraser, I.G. and Ranney, D.A. (1984). Male and female differences in enzyme activities of energy metabolism in vastus lateralis muscle. Journal of Neurological Science, 65, 323-331.
Hazelhurst, L.T. and Claassen, N. (2006). Gender differences in the sweat response during spinning exercise. Journal of Strength and Conditioning Research, 20, 723-724.
Hicks, A.L., Kent-Braun, J. and Ditor, D.S. (2001). Sex differences in human skeletal muscle fatigue. Exercise Sport and Science Review, 29, 109-112.
Hunter, S.K. et al. (2004). Men are more fatigable than strength-matched women when performing intermittent submaximal contractions. Journal of Applied Physiology, 96, 2125-2132.
Kendrick, Z.V. and Ellis, G.S. (1991). Effect of estradiol on tissue glycogen metabolism and lipid availability in exercised male rats. Journal of Applied Physiology, 71, 1694-1699.
Kjaer, M. (1998). Adrenal medulla and exercise training. European Journal of Applied Physiology and Occupational Physiology, 77, 195-199.
Komi, P.V. and Karlsson, J. (1978). Skeletal muscle fibre types, enzyme activities and physical performance in young males and females. Acta Physiologica Scandinavia, 103, 210-218.
Porcar, J., Bryant, C. and Comana, F. (2015). Exercise Physiology. Philadelphia, Pa.: F.A. Davis Company
Rooney, T.P. et al. (1993). Effect of estradiol on the temporal pattern of exercise-induced tissue glycogen depletion in male rats. Journal of Applied Physiology, 75, 1502-1506.
Salvador, E.P. et al. (2009). Effect of eight weeks of strength training on fatigue resistance in men and women. Isokinetics and Exercise Science, 17, 101-106.
Simoneau, J.A. and Bouchard, C. (1989). Human variation in skeletal muscle fiber-type proportion and enzyme activity. American Journal of Physiology, 257, E576-E572.
Tarnopolsky, M.A. (2000). Gender differences in substrate metabolism during endurance exercise. Canadian Journal of Applied Physiology, 25, 312-327.
Tarnopolsky, M.A. et al. (1997). Postexercise protein-carbohydrate and carbohydrate supplements increase muscle glycogen in men and women. Journal of Applied Physiology, 83, 1877-1885.
Tarnopolsky, M.A. et al. (1995). Carbohydrate loading and metabolism during exercise in men and women. Journal of Applied Physiology, 78, 1360-1368.
Thomas, G.A. et al. (2007). Maximal power at different percentages of one repetition maximum: influence of resistance and gender. Journal of Strength and Conditioning Research, 21, 336-342.
Zouhal, H. et al. (2008). Catecholamines and the effects of exercise, training and